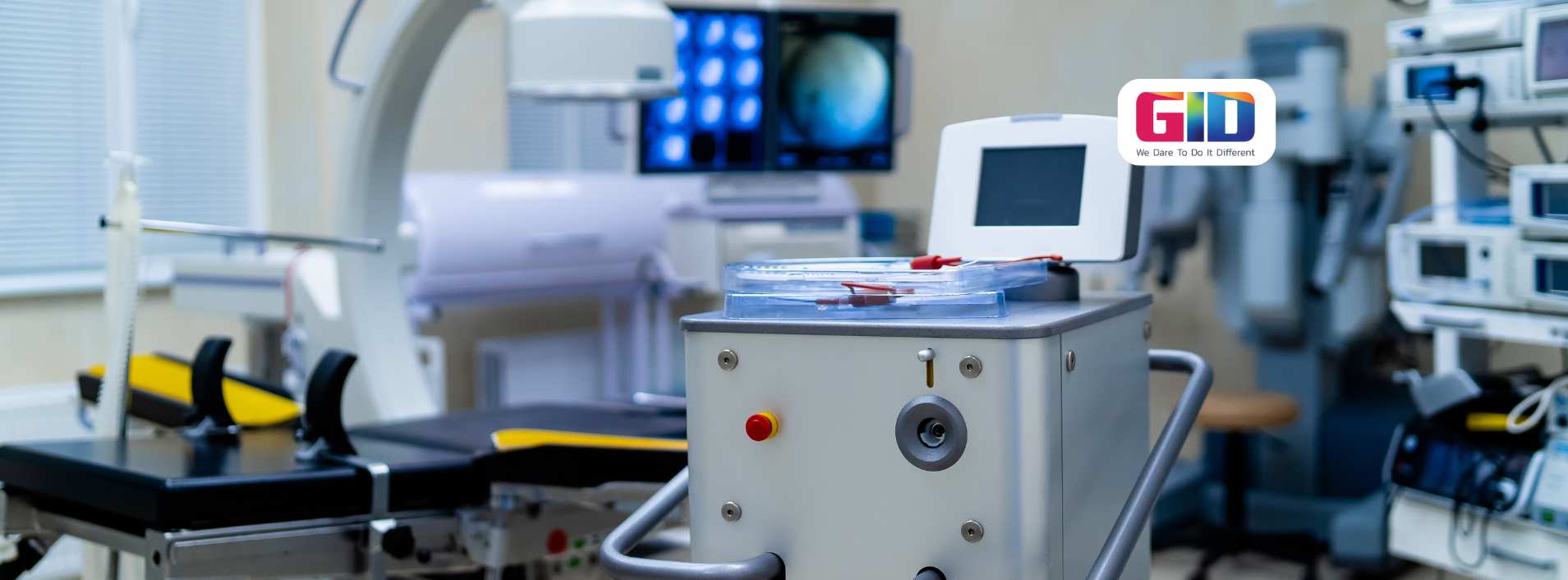
Developing a medical device product is always a challenging, time-consuming, and arduous task. However, developing a medical device product that includes software (products with embedded software), is an even more demanding task.
Today’s medical device products with embedded software can save lives, but there is still room for more innovative medical device products. Getting a new, innovative medical device product with embedded software into the market is certainly very lucrative, but more importantly, a revolutionary medical product can change the face of the healthcare industry by providing better healthcare treatments and services.
If you have a ground-breaking idea for a new medical device product with embedded software, then you should consider bringing it to life. If you proceed carefully, you will certainly achieve success with your conceived product. Before you start with medical device development, however, you should know about the four important things.
Is it a medical device?
Before you initiate the medical product development, you need to first determine whether your conceived product will be considered a medical device or not. As per the FDA (Food and Drug Administration), “Medical devices range from simple tongue depressors and bedpans to complex programmable pacemakers with micro-chip technology and laser surgical devices [sic].”
Therefore, a simple tongue depressor or a very complex CAT (Computerized Axial Tomography) scanner is a medical device, rather a medical device with embedded software in the latter case, but an activity tracker, like Fitbit’s wristband, is not a medical device. For that reason, it is vital to first find out how your conceived product will be classified. You need to know whether your conceived product can cause any harm. You also need to find out whether there are any safety risks involved.
If you are developing a medical device product with embedded software, knowing how your product will be classified will certainly have a major impact on the product development process, affecting factors such as assessing the development costs and timeline, creating the documents required for FDA approval, and testing the product.
Is your project scope defined accurately?
If you do not know the limits of the complexity of the project, software needs, and security requirements, you may underrate or overrate the product development efforts required. Will your medical device need complex mechanical components to function, or does it need to upload data to the servers to process and generate reports?
When you are trying to ascertain the complexity of your project, think about how many components the conceived medical device product might have. This will help define the timeline and cost of the project. Also, for the software part, you will need to get a clean user interface, which is easy to understand; hence, spend some time understanding the interface part. When defining the scope, be sure to take into account the complexity of the user interface development.
Modern, complex medical device products with embedded software are frequently supported by mobile applications, which manage medical device products with embedded software via smartphones and tablets. If your medical device product with embedded software requires mobile apps, be sure to clearly outline this in your scope.
Do you have any plans for the maintenance?
What is your plan for maintaining the software aspect of your conceived medical product? The FDA in its guidelines includes the corrective and preventive maintenance of the software. Having a plan for the maintenance will factor toward the development efforts and the long-term success of your product. If the new version of the OS (operating system) released, how the software of your product will be updated? Will the update affect the functionality of your product? Will it improve the performance and fix software bugs if there are any?
In developing a Medical Device Product with Embedded Software, crafting a plan around these questions is crucial for a Medical Device Product with Embedded Software. It ensures that your Medical Device Product with Embedded Software is both effective and compliant.
Do you need a product development partner?
Developing a medical device product with embedded software is no easy task; missing any step could lead to costly delays. Every detail must be flawless in the development of a medical device product with embedded software. Partnering with a dependable developer for your medical device product with embedded software can make a significant difference and be highly beneficial.
Have a complex product idea in mind, like a medical device product with embedded software? Want to bring your idea to life? Get in touch with vastly experienced industrial engineers at GID Development Corporation.
GID Company is one of the top product development companies in the US. They provide agile and economical product design, rapid prototyping, and product development services to inventors throughout the US and across the globe. Utilizing their vast experience and technology like the 3DEXPERIENCE Platform, which features BIOVIA, CATIA V6, SIMULIA, etc., the skilled industrial engineers at GID Development Corporation can help design, prototype, and develop medical device products with embedded software, electromechanical products, sports products, and all sorts of complex products.
If you are an Inventor, a Startup, or an Established Company that wants to turn your product idea into a profitable market-ready product then contact GID Company today!
As a leading Industrial Product Design and Development Company, we serve different industries and offer the best services for Electronic Product Design, IoT Product Design, Life Safety Product Design, Medical Device Design, and Mechanical Design.
You can also follow our blog to stay updated with the latest trends related to product design and development. Feel free to Contact Us or call us at +1714-323-1052.